Please note: the content of this website is now considered as archived.
We have not updated the information since 2016.
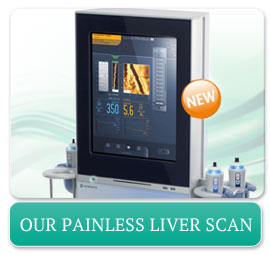
Painless Fibroscan instead of Liver Biopsy
Ruane Medical is the first clinic in Los Angeles to use the FibroScan®502 Touch. The FibroScan®502 Touch is an FDA approved device to study the liver directly for any signs of scarring (cirrhosis) without having to use a needle.
The Fibroscan can measure the texture of the liver by using "shear wave" vibration to the liver through a blunt button applied to the chest wall. There are no needles involved. No pain.
Results from the FibroScan ® 502 Touch device are accepted by the FDA in clinical trials in place of liver biopsy (organ puncture with a needle).
Fibroscan of the liver is not the same as ultrasound of the liver. Ruane Medical welcomes the FibroScan®502 Touch device as a genuine advance in the care of patients with Hepatitis C.